A variety of driver genes are present in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and those with high mutation frequencies include SF3B1, NOTCH1, and TP53, which are involved in the development of CLL/SLL and are associated with poor prognosis. The biological significance of some driver genes, such as DDX3X, has not yet been elucidated. DDX3X is located on the X chromosome. As one of the members of the DEAD-box helicase family, it is expressed ubiquitously in human tissues and participates in many biological processes. Here, we described the mapping of the DDX3X abnormalities and demonstrated its clinical significance in CLL/SLL. We also explored the effect and mechanism of DDX3X dysregulation on tumorigenesis and development of CLL by in vitro experiments.
To examine the mutational landscape of DDX3X, we performed next-generation sequencing on 402 CLL/SLL patients, and 17 patients (4.2%) had DDX3X mutations. Six patients were frameshift mutations and 2 were nonsense mutation, which caused truncation of DDX3X. Among the 7 cases of missense mutations, 4 cases involved the ATP-binding or C-terminal domain, which may affect the function of DDX3X. The remaining two cases are deletion mutation and splicing mutation. Next, we evaluated the clinical significance and prognostic role of DDX3X mutation. The patients with DDX3X mutations are mostly male (94.1% vs 66.5%, P=0.017), and had more adverse prognostic indicators: unmutated IGHV (88.2% vs 48.6%, P=0.002), 17p deletion (42.9% vs 11.4%, P=0.041), and 11q deletion (46.7% vs 15.7%, P=0.006). DDX3X mutation was an independent risk factor for disease specific survival of CLL/SLL in the competing-risk regression. Also, we found all (14/14) the mutated patients and 15.2% (5/33) of the non-mutated patients had low expression of DDX3X in bone marrow samples by immunohistochemistry. Overall, these results indicates that dysregulation of DDX3X are common in CLL/SLL and may contribute to its progression.
To delineate the functional consequences of DDX3X loss in CLL, we used CRISPR/cas9 system to construct stable DDX3X knockout (DDX3X-KO) MEC-1 and JVM-3 cells. DDX3X-KO cells showed significantly increased proliferation and reduced spontaneous apoptosis compared to vector control. Additionally, DDX3X-KO cells showed higher rates of cell viability and reduced apoptosis after treatment of ibrutinib or venetoclax. We further found that DDX3X-KO cells had higher expression level of phosphorylated BTK and MCL-1, which may explain the resistance to ibrutinib and venetoclax, respectively. Moreover, DDX3X-KO cells showed increased expression level of CXCR4 and enhanced chemotaxis in response to CXCL-12. In summary, loss of DDX3X function promotes progression of CLL by mediating more invasive biological behaviors of CLL cells.
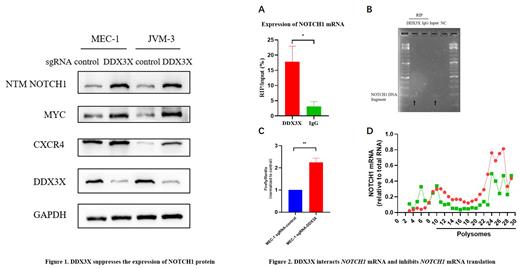
To evaluate the mechanisms by which DDX3X deletion affects CLL, we detected key signaling pathways in the tumorigenesis and development of CLL in DDX3X-KO and control cells. We found increased expression of NOTCH1 and its downstream target proteins in DDX3X-KO cells (Figure 1), indicating that loss of DDX3X activated the NOTCH1 pathway. Considering that DDX3X is an RNA-binding protein and mainly regulates translation, we speculated that DDX3X may regulate the translation of NOTCH1. We performed RNA binding protein immunoprecipitation assay and found that DDX3X directly interacted with NOTCH1 mRNA (Figure 2A, B). Dual-luciferase reporter assay showed enhanced activity of NOTCH1 5‘ UTR in DDX3X-KO cells (Figure 2C), further suggesting that DDX3X may regulate translation of NOTCH1 mRNA through binding to the 5‘ UTR. Finally, polysome profiling demonstrated that DDX3X knockout resulted in increased NOTCH1 mRNAs in polysome fractions (Figure 2D), confirming that loss of DDX3X function facilitates NOTCH1 mRNA translation.
In conclusion, DDX3X abnormalities are common in CLL and associated with poor prognosis. DDX3X dysregulation is involved in the progression of CLL by facilitating NOTCH1 mRNA translation through binding to the 5' UTR.
Disclosures
No relevant conflicts of interest to declare.